Research terms explained: glossary
When reading about or taking part in research, you might come across some unfamiliar terms.
Working with research volunteers, we've complied this list of definitions based on things they've come across.
Terms are listed in alphabetical order. If you feel anything is missing, please get in touch with the Research team at [email protected].
Adverse event
If a person taking part in a clinical trial, for example a drug trial, experiences any undesirable or unintended effects, it's described as an adverse event, even if it's not thought to be directly related to the drug or trial.
Blind
If a research trial is described as blind, it means that the people taking part don't know which study group they're in. For example, they don't know whether they're receiving the active treatment, or whether they're receiving a placebo. The researchers, however, do know which treatment each person is receiving.
Chief Investigator (CI)
The Chief Investigator of a research study is the lead researcher. They're responsible for the conduct of the whole clinical trial across the UK. They're also responsible for designing the clinical trial protocol and reporting the results of the study.
Clinical trials
Clinical trials are research studies that involve people. These studies help to understand the safety and impact of a treatment or therapy for a specific group of people, as well as look for any potential side effects.
Control
In clinical trials there will often be a control group. This group acts as the comparison group. Rather than receiving the specific treatment under investigation, the control group will receive either another treatment, no treatment at all, or a placebo. Control groups are sometimes age-matched to the treatment group, which means the participants are all of a similar age.
Researchers will monitor everyone in the same way to then compare the results. This helps show whether the treatment under investigation actually works, and how effective it is.
Crossover trial
A crossover trial is a type of clinical trial in which the participants receive more than 1 drug or therapy, and the order in which they take them depends on which group they're in.
Participants are randomly split into 2 groups. Each group takes a different drug or therapy for the first half of the trial. There is then a period of time where neither group takes a drug, called a wash-out period. For the second half of the trial, the groups switch to the drug they haven't taken yet.
Crossover trials are beneficial because they allow researchers to test 2 or more interventions in 1 study. Fewer participants are needed to test both interventions, and it can be a quicker way of testing more than 1 drug or therapy.
Double-blind
If a research trial is described as double-blind, it means that neither the people taking part nor the researchers know who's receiving the active treatment or therapy versus the placebo. This is the gold standard in research because it means that any predetermined beliefs about the treatment that the researchers or participants may have don't affect the overall outcome of the trial.
Efficacy
Efficacy describes how well a drug works to produce the desired effects in research settings, such as a clinical trial. If a drug works well in a trial, the drug is effective and is said to have efficacy.
Eligibility criteria
All research trials will have eligibility criteria to help guide who can take part in a specific study and who can't. These criteria will have been carefully considered when the research was planned to make sure certain characteristics and factors don't interfere with the study for safety reasons.
It can include a range of factors including age, length of time since diagnosis, whether you've received other treatments (such as deep brain stimulation) or medication, or whether you experience other health conditions. Early stage research, such as phase 1 and 2 clinical trials, often have stricter eligibility criteria for safety reasons.
Ethics
Research ethics are a set of principles which must be followed to ensure that a research project is ethical and safe. This will involve considering whether a project is necessary or justified, whether the drug poses a risk to participants, whether the researchers are qualified to carry out the research, and whether there is enough financial support to complete the research.
You can learn more about keeping everyone safe in research in a short National Institute for Health and Care Research (NIHR) video. Watch the video on YouTube.
Gold standard
The gold standard in research is the method that is widely agreed to be the best and most accurate way to test a new drug or treatment. In Parkinson's research, the gold standard method is often a double-blind, randomised trial, with a placebo group.
Healthy volunteer
Healthy volunteers are people who want to take part in research but who don't have the health condition that the new treatment or therapy is being investigated for. In terms of Parkinson's research, healthy volunteers don't have a Parkinson's diagnosis.
In the very early stages of testing new drugs, the drug will be tested on healthy volunteers before it's tested on people with a specific health condition, who may be more vulnerable.
Health Research Authority (HRA)
The HRA protects and promotes the interests of patients and the public in health research. Any research project that involves recruitment of NHS patients, staff, premises, resources or data or tissue in England must go through the HRA approval process.
Informed consent
A person must give their permission before they receive any type of treatment or take part in a research trial. Informed consent means that a person has been fully informed and understands what the treatment or research is. They must fully understand what they're consenting to in order to give informed consent. People can withdraw their consent and stop taking part in a study at any point.
Intervention
In clinical trials, the intervention is the potential drug, wearable or device, therapy, activity or programme that's being investigated for a condition.
Multi-arm, multi-stage study design
This type of design means that several different treatments can be tested all at once. This means treatments can be assessed at earlier timepoints to see whether or not they appear to be effective before deciding to take them all the way to the end of the trial. If a treatment doesn't appear to be effective, it will be removed from the trial and replaced with another potential treatment, without the need to close everything down and then re-set it all up again.
This type of study has lots of advantages – it means we get answers on multiple different treatments all within 1 trial, and a smaller percentage of people need to be on the placebo arm. It also speeds up the study process compared to normal trials, saving time and money.
Medicines and Healthcare products Regulatory Agency (MHRA)
The MHRA is an Executive Agency of the Department of Health and Social Care. It's responsible for protecting and promoting public health and patient safety. It ensures that medicines, healthcare products and medical equipment meet appropriate standards of safety, quality, performance and effectiveness, and are used safely.
On and off time
People with Parkinson's experience fluctuations in their symptoms, which are sometimes described as on and off times. On time refers to the period of time during which a person's symptoms are well controlled by medication. Off time refers to the time between medication where symptoms aren't being managed as effectively.
Phil shared his experience of off time before taking part in the produodopa trial: "Whilst being off meds isn't nice, it's a necessary evil for some (but not all) of the research that I have taken part in. I wouldn't let it stop me from taking part in research in the future."
Read more about Phil's experience of off time in our research blog.
Oral administration (of a drug)
Oral administration means that a drug is taken through the mouth, swallowed and then enters the stomach. This can involve swallowing the medication with a liquid, such as water, to make the process more manageable. Medication will often be in a capsule or pill form if it's to be taken orally.
Outcome measure
Outcome measures are how the researchers will define the success of a study. These are predefined before a research study even begins. For example, the main outcome could be to show that the intervention is safe and then secondary to this, effective at improving a certain symptom. The outcomes are usually referred to as primary, secondary or exploratory.
Participation information sheet (PIS)
Before you take part in any research study, you'll be given a PIS. This is an important document that gives you all the information you'll need to give informed consent to take part in the research study.
The PIS will give you a brief summary of the research project and explain what the aims of the study are. It will also explain what taking part will look like, including what you'll be asked to do, how long the study will take, and outline any risks and benefits of taking part. It should be written in an easy-to-understand way.
It should also include contact details for the researcher. We encourage you to contact them and open this line of communication if you have questions.
Pharmacodynamics
Pharmacodynamics is the study of how drugs have effects on the body. It explores the chemical, physiological and molecular effects of drugs on the human body. It's a branch of pharmacology.
Pharmacokinetics
Pharmacokinetics is the study of what the body does to a drug, or the fate of a drug once within the body. It explores how a drug is absorbed, distributed, broken down and excreted by the body. It's a branch of pharmacology.
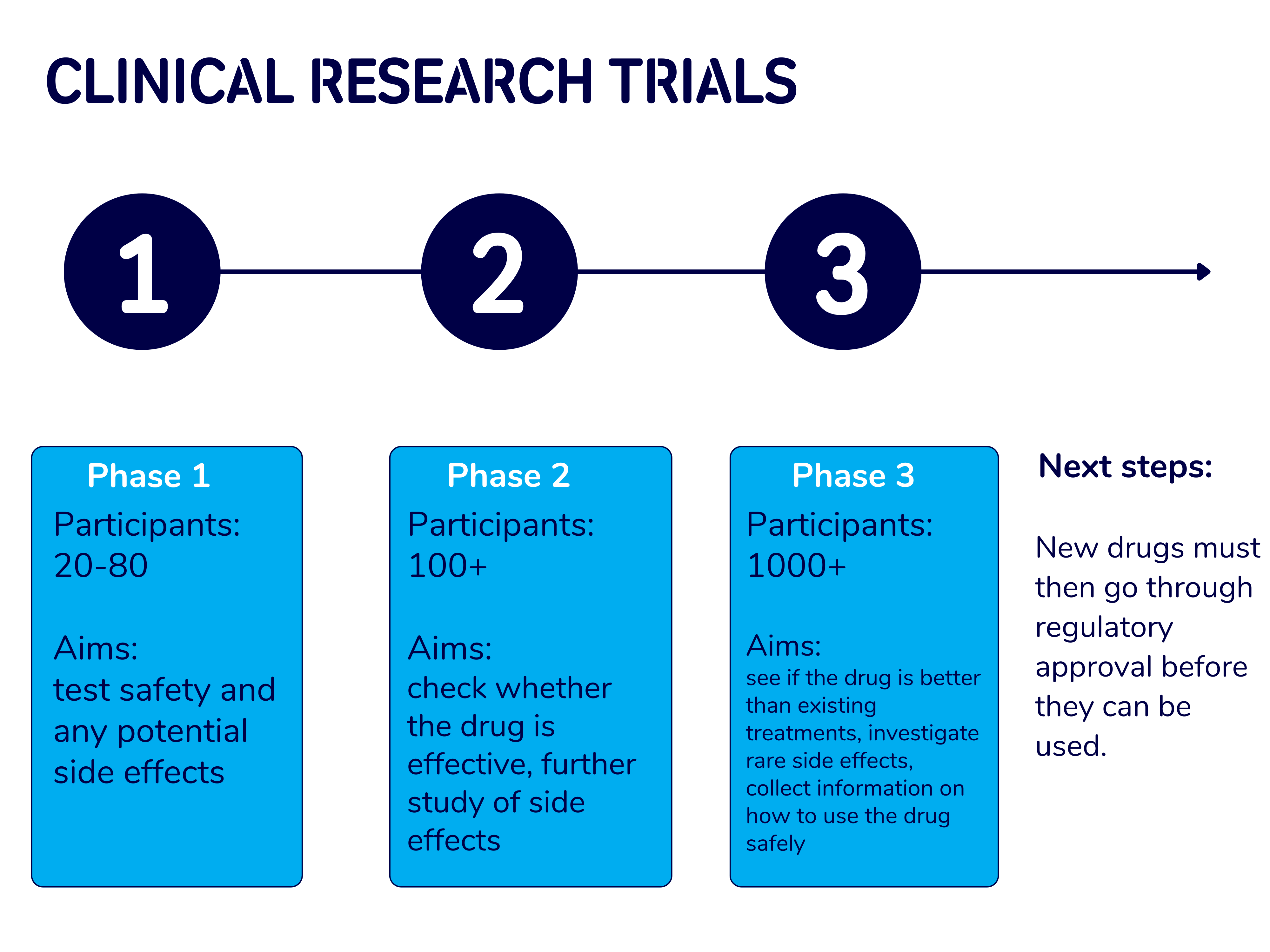
Phase 1, phase 2 and phase 3 clinical trials
Clinical trials are divided into phases:
- Phase 1 are small trials that aim to see whether a new treatment is safe and check for any side effects.
- Phase 2 trials are much larger than a phase 1 trial. A phase 2 trial will aim to find the correct dose of a drug, look at potential side effects, and see how well the drug works in a larger group of people.
- Phase 3 trials are large and involve hundreds of people. They aim to find out even more about how well the new treatment works and will usually compare the treatment to a control, which is often a placebo.
Find out more about these phases in our research blog.
Placebo
A placebo is a treatment that appears to be identical to the active treatment under investigation, but is designed to have no effect or benefit. It might also be referred to as a dummy or sham treatment. In research studies, participants are split into 2 groups and will receive either the real treatment or a placebo.
Participants and researchers often don't know who's receiving the placebo vs the active treatment. The effects of the treatment being investigated can then be compared to the effects of the placebo to see whether the treatment really works.
Desmond and Maria shared their experience of when Desmond was considering being part of a drug trial. Maria said: "One question was that Desmond might not receive the drug, but instead receive the placebo (dummy pill). So he may not see any benefit himself being part of the trial. But having discussed it, we decided it wasn't a worry for us.
"We felt that even if it didn't work for Desmond, it might mean that someone else doesn't have to go through what he's going through."
Placebo effect
The placebo effect is a phenomenon where participants in a trial who are receiving the placebo (a dummy treatment), report or show improvements despite nothing having changed. In a controlled study, participants don't know whether they're receiving the placebo or active treatment. The placebo effect is explained by the person's belief in the treatment and their expectation of feeling better.
Principal Investigator (PI)
The PI of a research study is the lead researcher at a specific research site. At the research site, the PI is responsible for all aspects of the clinical trial, and will oversee the trial as it runs.
Sometimes a clinical trial will be taking place across multiple sites, particularly if it has lots of participants and having multiple sites increases the ability of people to take part. If there is only 1 research site, the Chief Investigator and Principal Investigator are often the same person.
Protocol
All clinical trials follow a plan which is called a protocol. The protocol describes how the clinical trial will be conducted, including the research background, goals, design, method and eligibility criteria.
Randomised
A randomised study means that participants were randomly assigned to the different study groups, for example the treatment group or the control group. It means that each participant has an equal chance of receiving the active treatment.
With special thanks to our Research Participation Steering Group members and Alan Davey of the Parkinson's UK Havering and District Branch for their work putting this glossary together.

