Dialling down inflammation to protect brain cells in Parkinson’s
We’re investing in drugs that reduce harmful inflammation. But what is inflammation and why could stopping it provide a vital new treatment?
What is inflammation?
Inflammation is a process that is vital for defending the body against harm from things like infections, injuries, and toxins.
When something damages our cells, they release tiny messenger proteins to alert the immune system that something is wrong.
It’s the equivalent of a 999 call to our immune cells, which constantly patrol the body, to come to the rescue.
Inflammation happens all over the body wherever a threat is detected. While you might be more familiar with the outcome of a cut on your finger, and the pain, swelling, redness and heat that it may cause, inflammation also occurs in other parts of the body that are not so easy to see.
In the brain, the cells that respond to inflammation are called microglia.
Microglia make up 10 to 15% of all cells found within the brain and are its main line of defence. Under normal conditions, microglia sit around monitoring the environment for signs of trouble, such as inflammation.
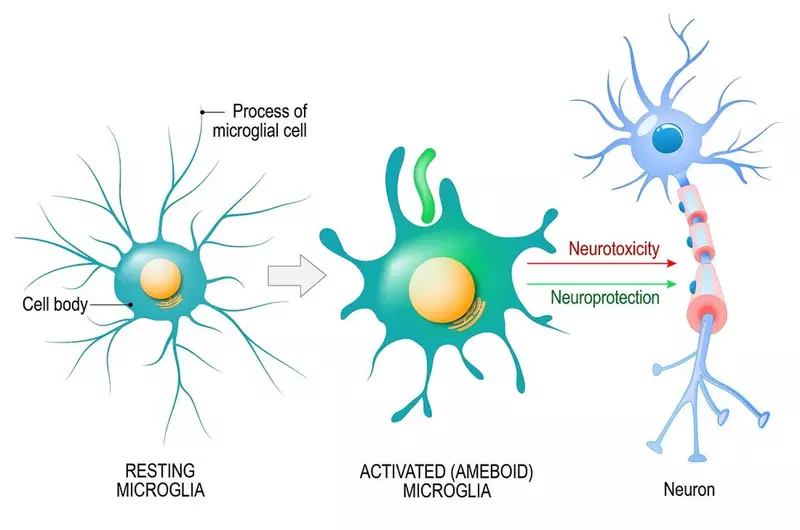
When they detect inflammation, microglia switch into 'attack' mode and rush to the site of infection or injury.
Once there, microglia look for what’s causing the inflammation. This could be foreign invaders like bacteria, or dead or dying brain cells, or damaged proteins. They release chemicals to destroy the offenders and act as a call for reinforcements, attracting more microglia to join in.
After they have destroyed the invaders or removed damaged cells, they switch off attack mode, hoover up the resulting debris and release chemicals to promote healing and repair.
However, in some cases, microglia may remain stuck in ‘attack’ mode and this seems to be more likely to happen as we age.
This kind of constant (or 'chronic') inflammation can be damaging to our own cells. Chronic inflammation in other parts of the body has been linked with the development of a range of different conditions including cancer, heart disease, and rheumatoid arthritis.
It’s now becoming clear that chronic inflammation inside the brain may be involved in neurodegenerative conditions including Parkinson’s and Alzheimer’s.
We talked to our Associate Director of Research, Professor David Dexter, to learn more about how inflammation might be involved in the development of Parkinson’s. And how new drug treatments might help.

What do we know so far about inflammation in Parkinson’s?
"There is growing evidence that excessive inflammation happens in Parkinson’s and that stopping it could be beneficial.
"Studies looking at post-mortem brain tissue show that there are many more microglia present in the brain areas affected by Parkinson’s, these microglia are in an activated (attack) state and we can also see that they are releasing inflammatory chemicals.
"In pretty much all animal models, these are usually rats or mice that have either been genetically altered or treated with chemicals to induce Parkinson’s-like damage in the brain, we see elevated inflammation. And experiments have shown that giving drugs with anti-inflammatory properties to these animals can slow the spread of damage.
"In people with Parkinson’s, we can detect signs of inflammation in blood samples. And crucially, recent studies have shown that people with faster progressing or more aggressive forms of the condition seem to have the highest levels of inflammatory markers.
"Finally, in large-scale epidemiological studies which look at the health records and outcomes of thousands of people, scientists have found that people who take existing anti-inflammatory drugs like ibuprofen on an intermittent basis throughout their lives are less likely to develop Parkinson’s than those who use them rarely. This suggests that anti-inflammatory medications may have a protective effect against Parkinson’s."
And what don’t we know?
"I think it’s fair to say that we’re still just scratching the surface. Inflammation is an incredibly complex process at the best of times, and when you combine that with the complexity of a condition like Parkinson’s things become even more difficult to unravel.
"We still have a long way to go to understand how inflammation gets out of control in Parkinson’s, and there are a number of big questions that I believe we need to answer.
"One of these is about timing. We know that the inflammatory response has different phases. What we don’t know is when inflammation tips over from helpful to harmful and therefore when the right time to intervene might be. If we give these drugs at the wrong time we could stop microglia from doing a job that needs doing, but if we’re too late they may not be effective. We need to identify the right window of opportunity.
"Another is where does inflammation start? There is now lots of interesting research that suggests it could be in the gut. We know that people with Parkinson’s seem to have a different blend of bacteria living in their digestive system than people without the condition. Emerging evidence suggests that people with the condition may have more bacterial species that trigger an inflammatory response. It’s possible that the start of inflammation could be here rather than in the brain."
So, from what we know right now, how do you think inflammation might be involved in Parkinson’s? And what impact could drugs that reduce this inflammation potentially have?
"My feeling is that the chronic inflammation we see in Parkinson’s is like pushing down on the accelerator pedal.
"At the start, brain cells experiencing damage from Parkinson’s send out distress signals and may also be leaking abnormal proteins like alpha-synuclein into the brain. These signals attract the attention of microglia who come to try and help the damaged cells.
"However, for some reason, the microglia go too far. They start releasing chemicals that contribute to the damage to struggling brain cells, they call in reinforcements in the form of more microglia, and they may even destroy affected brain cells themselves.
"And once this process starts it’s like a runaway train that can’t be stopped, so surrounding brain cells get swept up in this toxic inflammatory process.
"I think we can feel fairly confident that we might be on the right track with targeting inflammation in Parkinson’s because of what we’ve seen in other conditions. There are already licensed drugs that target inflammation and are disease-modifying for multiple sclerosis. Drugs are also being developed to target inflammation for Alzheimer’s along very similar lines to those being developed for Parkinson’s. It really feels like momentum is building in this area.
"Importantly, researchers are now starting to be able to use new imaging techniques that can pick up microglia inside the brain. This means we can see where microglia are and also whether they are in an activated (attack) state, which will be crucial when we come to test new treatments to see if they are having the desired effect.
"If we’re right about the role of inflammation and we can develop drugs that stop this catastrophic process they have the potential to significantly slow the condition. If we can slow the progression from a march to a crawl that would be absolutely transformative, especially if it can be coupled with drugs that tackle other processes involved in brain cell death."
Creating new drugs to target inflammation
There are already drugs available to reduce inflammation, things like ibuprofen, aspirin and steroids.
However, these drugs don’t directly target the processes involved in inflammation in Parkinson’s and they can also have serious side effects if they are taken long term.
So with current drugs unlikely to be the answer to inflammation in Parkinson’s, we need to create new ones.
That’s why Parkinson’s UK invested £150,000 in 2020 through its Virtual Biotech programme to kick off a project to design new drugs that specifically target inflammation in Parkinson’s.
This initial work has identified a family of molecules which show great potential, and the charity is now committing to invest up to £3m to accelerate their development.
Dr Richard Morphy, Drug Discovery Manager at Parkinson’s and leader of the project, explains further:
"We are focusing on developing molecules that can get into the brain and target a specific protein on the surface of microglia.
"This particular protein seems to be present at significantly higher levels in the brains of people with Parkinson’s than in people without the condition. There is also evidence that interactions between this protein and alpha-synuclein, a protein which goes rogue in Parkinson’s, increase microglial activation.
"This suggests that this microglia protein could be central to the runaway inflammation seen in Parkinson’s and drugs that block it may provide a breakthrough new anti-inflammatory treatment.
"Over the past year, we’ve developed a new family of potential drug molecules which looks very promising. That’s why we’re now investing a significant amount, up to £3m, to rapidly drive forward work to turn these molecules into drugs that can be taken forward into clinical trials in people with Parkinson’s."
About the Parkinson’s Virtual Biotech
Parkinson’s UK will invest up to £3m over the next 30 months in this pioneering project.
The funding comes via the Parkinson’s Virtual Biotech, the charity’s drug development arm which fast-tracks the development of treatments to transform the lives of people with Parkinson’s.
This unique programme is supporting the development of 9 important projects which are all accelerating progress towards vital new therapies, including 3 active clinical trials.
The goal is to deliver life-changing new treatments to the Parkinson’s community in the shortest possible time frame.
We can find a cure. We can't do it without you.
You can help us take the next steps to dial down inflammation in Parkinson's.
